
MRSi 2025: The Stars of ART
Abstract Information
Your Abstract could earn you a chance in the spotlight at the 2025 Midwest Reproductive Symposium international. Two awards will be given: one to the top in-training individual submission and the second to the overall top abstract (regardless of submitter status). The winner of each will give an oral presentation on Saturday morning in the Grand Ballroom. Abstracts selected for poster presentation will be featured at the Friday night Abstract Poster Session.
Application Deadline: 04/20/2025
Notification Date: 04/25/2025
Abstract Submissions are Now Closed
You must have a MRSi profile to submit your abstract.
You can create a profile here.
Accepted abstracts will be published in the North American Proceedings in Gynecology and Obstetrics.
APPLY FOR A SCHOLARSHIP - ATTEND MRSI FOR FREE!
Even if you aren't able to submit an abstract, Individuals in training (Fellows, residents, medical students), REI lab professionals, nurses and genetic counselors are eligible to apply for a scholarship which reimburse expenses for air / ground travel, hotel, and conference registration.
All others submitting abstracts will need to cover their own air/ground travel, hotel and conference registration.
ABSTRACT INFORMATION
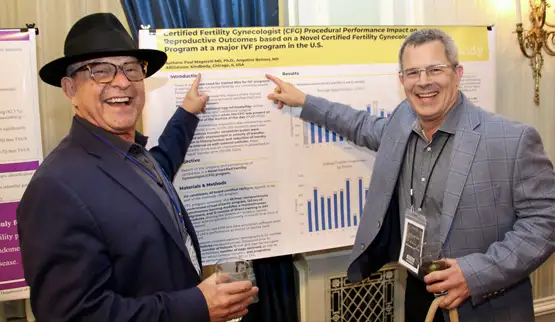
If the nominating committee chooses your abstract, you will be invited to showcase your poster to conference attendees. The poster session is scheduled for Friday June 6th at 5:45pm.
AWARDS
Two awards will be given: one to the top in-training individual submission and the second to the overall top abstract (regardless of submitter status). The winner of each will give an oral presentation. If the same individual wins both awards, they will only do one oral presentation.
You will need to submit a blind copy of your abstract with all personally identifying information removed which will be used for judging by our Nominating Committee. Results will be tabulated and all submitters will be notified of their abstracts status by April 30, 2023. Full details regarding the poster session and oral presentations will be given at that time.
FORMAT GUIDELINES
- Content -Abstracts must adequately describe the research performed so that the quality, originality, and completeness of the work can be evaluated. Abstracts submitted to previous conferences will only be accepted if they have been enhanced through the addition of new data, results, and/or interpretation. Only structured abstracts with the following headings can be submitted:
- Objective: An introductory sentence indicating the objective and purpose of the study
- Design: A briefly worded description of the study design
- Materials and Methods: A description of experimental procedures including applicable statistical evaluation
- Results: A summary of the new data and results
- Conclusions: A statement of the study's conclusions
- Support: Identify all sources of financial support for the research or state "None" if applicable
- Abbreviations used in abstracts must be defined. Abbreviations are permitted in titles if they immediately follow the term being abbreviated and are enclosed in parentheses. If used in the text, they should be defined at first mention if not already defined in the title.
- ACCME guidelines require generic names be used for pharmaceuticals, biologics, and medical devices. The trade name of the particular product used in a study may be included in parentheses the first time the product is referenced. The trade name may be used if the product is the only one of its general type and use of the generic name would encumber the reader.
- Limit the number of authors to six (6) - no exceptions.
- In the abstract text boxes, list the presenting author first. Type each authors' first name, initial, last name and affiliation in upper and lower case, in italics, not in bold. The authors' names will be listed in conference materials exactly as submitted.
- Do not identify individuals in the title or abstract body.
- Bold the title and type it in all CAPS.
- Abstract size limits - the body of the abstract is limited to 2,000 characters, which may include one table. Inclusion of a table will replace some character space. Figures and/or graphics are not permitted and will be deleted. Titles are limited to 200 characters and are not included in the abstract body character count. Author names and affiliations are not included in either character count.
- Review and selection of abstracts: Abstracts will be reviewed by the Program Nominating Committee and selected for inclusion in the program on the basis of scientific content and significance to the field of reproductive medicine.
- Authors assume complete responsibility for the integrity and validity of the data at the time of submission.
- Prepare your poster. The dimensions for the poster should be 3 feet tall by 6 feet wide. We will provide a display board that you will stand nearduring the reception. Posters can be dropped off in the Marquette Room upon your arrival at the Drake.
To submit your abstract, first create an MRSi profile account. If you have questions, please contact coordinators@mrsmeeting.org.

